Why Do Most Ionic Compounds Dissolve in Water
Ionic solids or salts contain positive and negative ions. Ionic compounds conduct electricity when molten liquid or in aqueous solution dissolved in water because their ions are free to move from place to place.
Unit 5 Double Replacement Replacements Ppt Video Online Download
9 What substances will dissolve in water polar or nonpolar.
. Ionic compounds are crystals with equal numbers of positive and negative ions. 4 Why do acids release hydrogen ions in water. Covalent compounds dissolve into molecules while ionic compounds dissolve into ions that conduct charge.
This is because water dissolves polar substances which is the consistency of the ionic compound whereas covalent compounds are non-polar. Likewise the partial negative charges on the oxygen atoms in water are attracted to the positively charged sodium ions. Many covalent molecules do dissociate in water HCl as pointed out in the comments phenol acetic acid for example whereas some ionic compounds do not to any appreciable extent eg.
Ionic compounds cannot conduct electricity when solid as their ions are held in fixed positions and cannot move. Snowflake is an example of compound that begins to form when an extremely cold-water droplet freezes into a pollen or dust particle in the sky. Because of the structure of water it is a polar molecule.
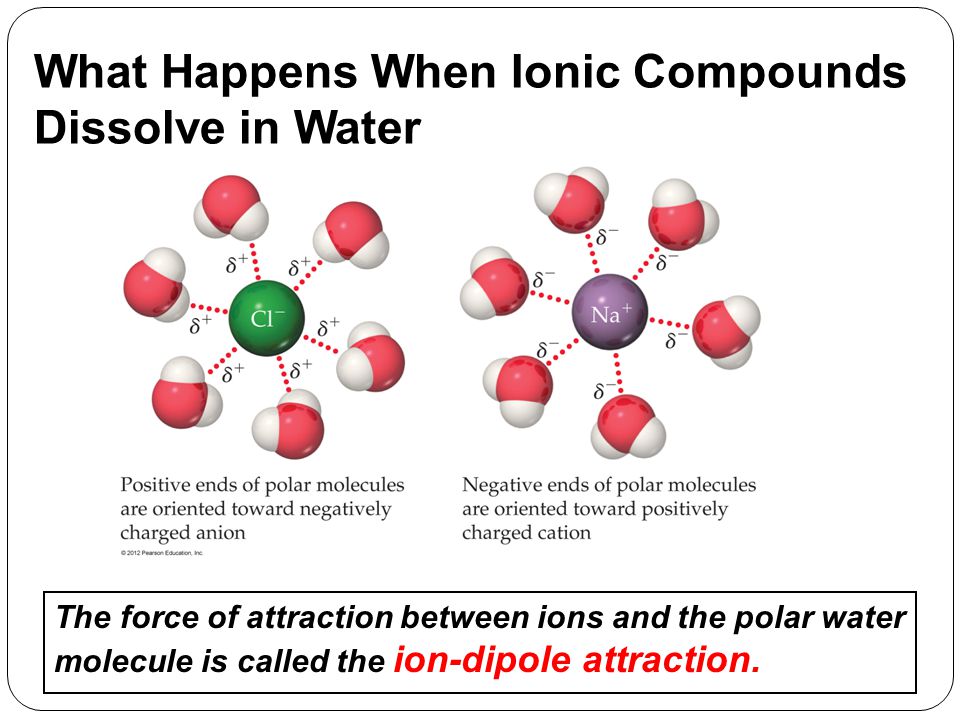
Ionic compounds are easily soluble in any liquid that is capable of breaking the ionic bond in them. This allows the ionic compound to dissolve whereby water molecules and ions intermingle and mix thoroughly. When an ionic compound dissolves in water the solution conducts electricity.
6 What is a substance dissolved in. Why do ionic compounds dissolve in water. Because everything tends to the lower energy state and dissolved ionic compounds are lower energy than undissolved.
The melting and boiling points of molecular compounds are generally quite low compared to those of ionic compounds. 6 What compounds Cannot dissolve in water. Hence can not dissolve them and they all have covalent bonds and.
11 What is the term for the process of a polar molecular compound dissolving in water and forming positive and negative ions. Mostly water dissolves ionic compounds because of the like dissolves like. Differences in electronegativity account for the partial positive charge carried by waters hydrogen atoms and the partial negative charge of its oxygen atoms.
1 Why do some ionic compounds not dissolve in water. When you place an ionic substance in water the water molecules attract the positive and negative. Then the negative charges of the compound are attracted to the positive charges of the water on the other hand.
Why are ionic compounds soluble in water. Silver chloride lead sulfate. That means that the hydrogen atoms have a slightly positive charge and the oxygen atom has a slightly negative charge.
Most ionic compounds dissolve in water because the process is thermodynamically favourable and kinetically accessible. Ionic However it should be noted that. Water molecules can attract most ions very well luring the ions away from each other.
Many other solvents such as kerosene and petrol are not capable of breaking the ionic bond. 3 Do polyatomic ions dissociate when dissolved in water. They do this by hydrating the ions.
Beside this why is an ionic compound soluble in water. Water breaks the ionic bond by hydrogen bonding as water itself has a more ionic bond and is polar in nature. Water is a polar solvent.
5 What ions are not soluble in water. The general reason is that the system that is molecule plus solvent will move into its lowest energy state. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent.
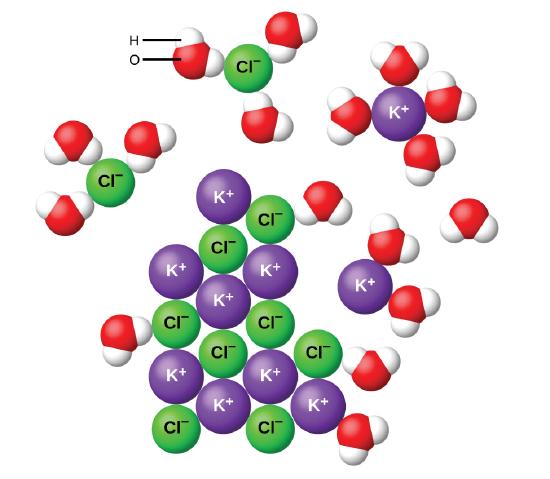
They do this by hydrating the ions. Ionic compounds dissolve in water because the hydrogen and oxygen atoms in the H2O molecules have partial charges that attract the ions in the solid compound causing it to dissociate into separated ions. Covalent compound cannot conduct electricity because their ions dont dissociate in water and hence they cannot freely.
To dissolve an ionic compound the water molecules must be able to stabilize the ions that result from breaking the ionic bond. 10 What ions are present when an alkali is dissolved in water. 4 When ionic compounds split up into individual ions in aqueous solution this process is called.
Covalent compounds do not dissolve in water since they are composed of non- polar molecules. 3 What type of ions do bases release. The first example that springs to mind is sodium chloride.
8 What substances when dissolved in water will conduct electricity. Ionic compounds are also more soluble in water than covalent compounds. Water is a polar molecule.
NaCl s aq NaCl aq ie. Ionic compounds dissolve in the presence of water because the positive components of the compound are attracted to the negative charges. Na aq Cl aq.
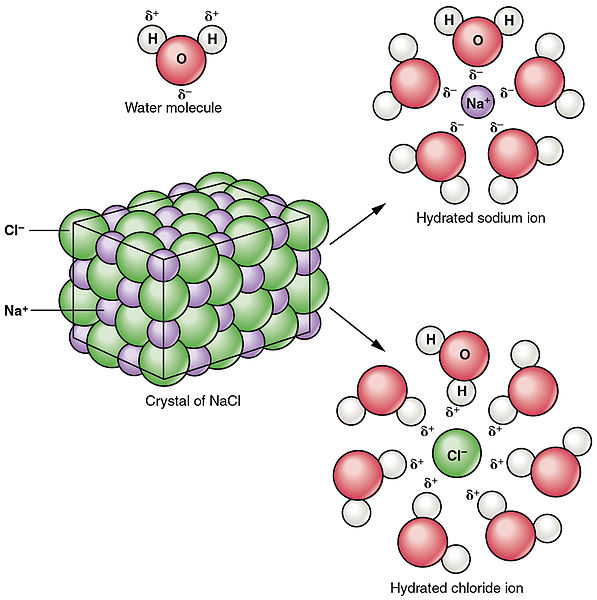
Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together the salt dissolves. Ionic compound cannot conduct electricity in solid state. 1 As A Group Compounds That Release Ions When They Dissolve In Water Are Called.
2 What will form individual ions when dissolved in water. Their ions will dissociates and this make tgemto freely move from one place to another. To dissolve an ionic compound the water molecules must be able to stabilize the ions that result.
Sodium chloride dissolves in water because it is a blank compound. This is because the energy required to disrupt the intermolecular forces between molecules is far less than the energy required to break the ionic bonds in a crystalline ionic compound Figure 62. In water the electrostatic forces of attraction between oppositely charged ions are overcome allowing the ions to dissociate and dissolve.
To dissolve an ionic compound the water molecules must be able to stabilize the ions that result from breaking the ionic bond. When a solute dissolves in water the process is referred to as. Ionic compounds can conduct electricity when they are dissolved in water or in liquid form.
2 What are compounds that release ions when they dissolve in water called. In the case of sugar and water this process works so well that up to 1800 grams of sucrose can dissolve in a liter of water. 5 What type of inorganic substances usually dissociate in water.
When you place an ionic substance in water the water molecules attract the positive and negative ions from the crystal. Example numbers not measured This shows a change in energy is negative ie costs no energy to make happen. Why do ionic compounds dissolve well in water.
Water is a polar molecule.
Are Ionic Compounds Soluble In Water Quora

Dissolving Process Chemistry For Non Majors

Solubility Do Now P 4 Remember Likes Dissolve Like Things That Dissolve In Water Soluble Ionic Acids Ex Hcl Bases Ex Naoh Polar Covalent Ppt Download
Ionic Bond Is Stronger Than Covalent But Nacl Ionic Bond Easily Break In Water Why Quora
Why Are Some Substances More Soluble And Some Are Less Soluble Quora

7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts

Aim How To Determine If An Ionic Solid Dissolves In Water Ppt Download

A Level What Happens When An Ionic Compound Dissolves In Water Energetics Lattice Enthalpy Enthalpy Of Hydration Solution Ks5 Gce Chemistry Revision Notes

Unit 5 Double Replacement Replacements Ppt Video Online Download

Solutions The Dissolving Process Lg I Can Explain The Behaviour Of Molecular And Ionic Compound In Water Ppt Download
What Determines Whether A Solid Is Soluble In Water Socratic

What Is Dissolving When An Ionic Compound Eg Salt Dissolves In Water The Compound Disassociates Breaks Apart Into Cations And Anions Ex Ca No3 2 S Ppt Download

Are Ionic Compounds Soluble In Water O Level Chemistry Notes

Why Are Ionic Compounds Soluble In Water Youtube

Why Are Ionic Compounds Soluble In Water When Ion Dipole Interaction Is Weaker Than Ionic Bond Quora

General Solution Chemistry Molarity Properties Of Solutions 1

Ionic Compounds And Metals Ppt Download

Solved What Happens When Ionic Compounds Dissolve In Water Chegg Com

Comments
Post a Comment